Join the thousands of HNF volunteers who are fueling CMT research. The more you share, the more we’ll learn. Complete your GRIN surveys today!
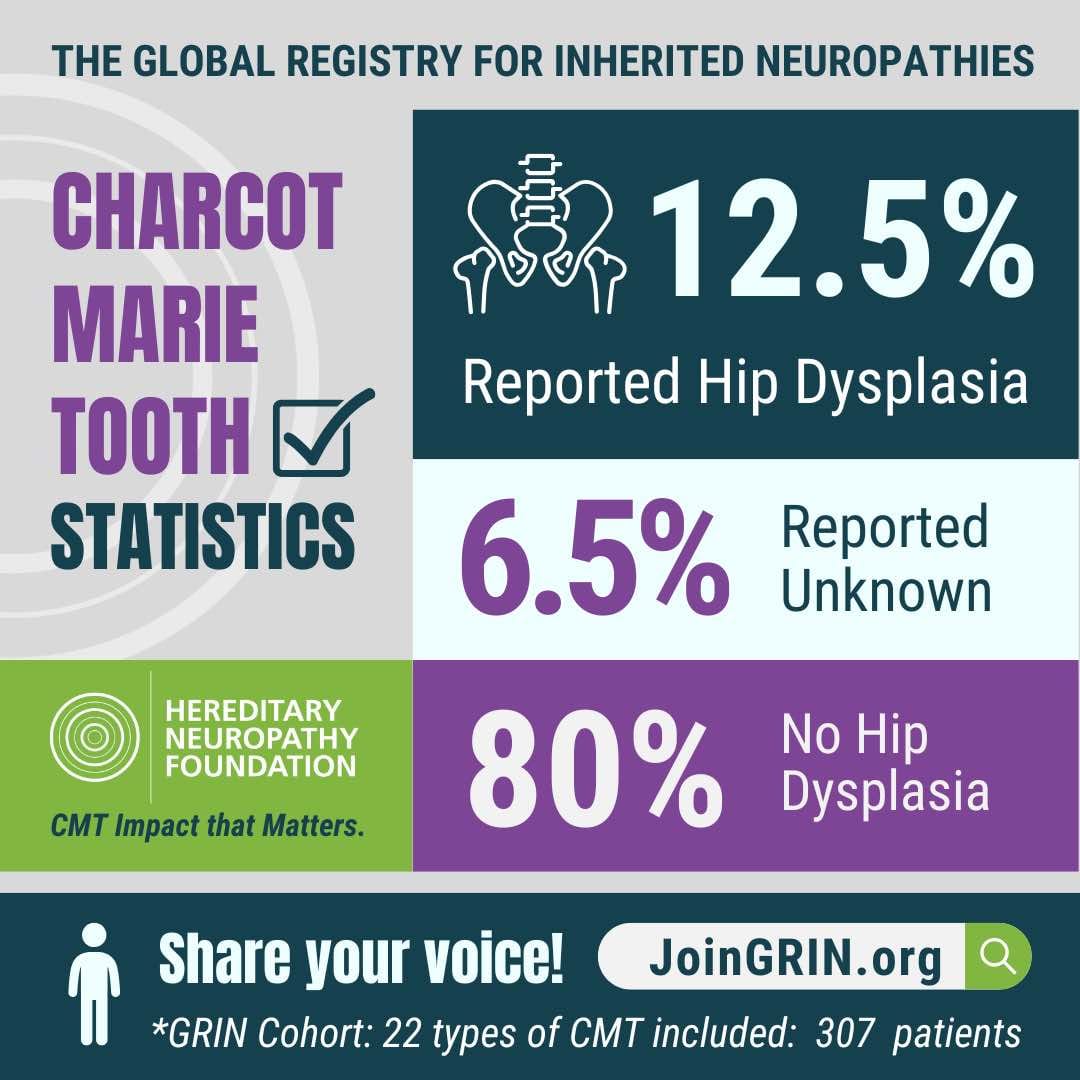
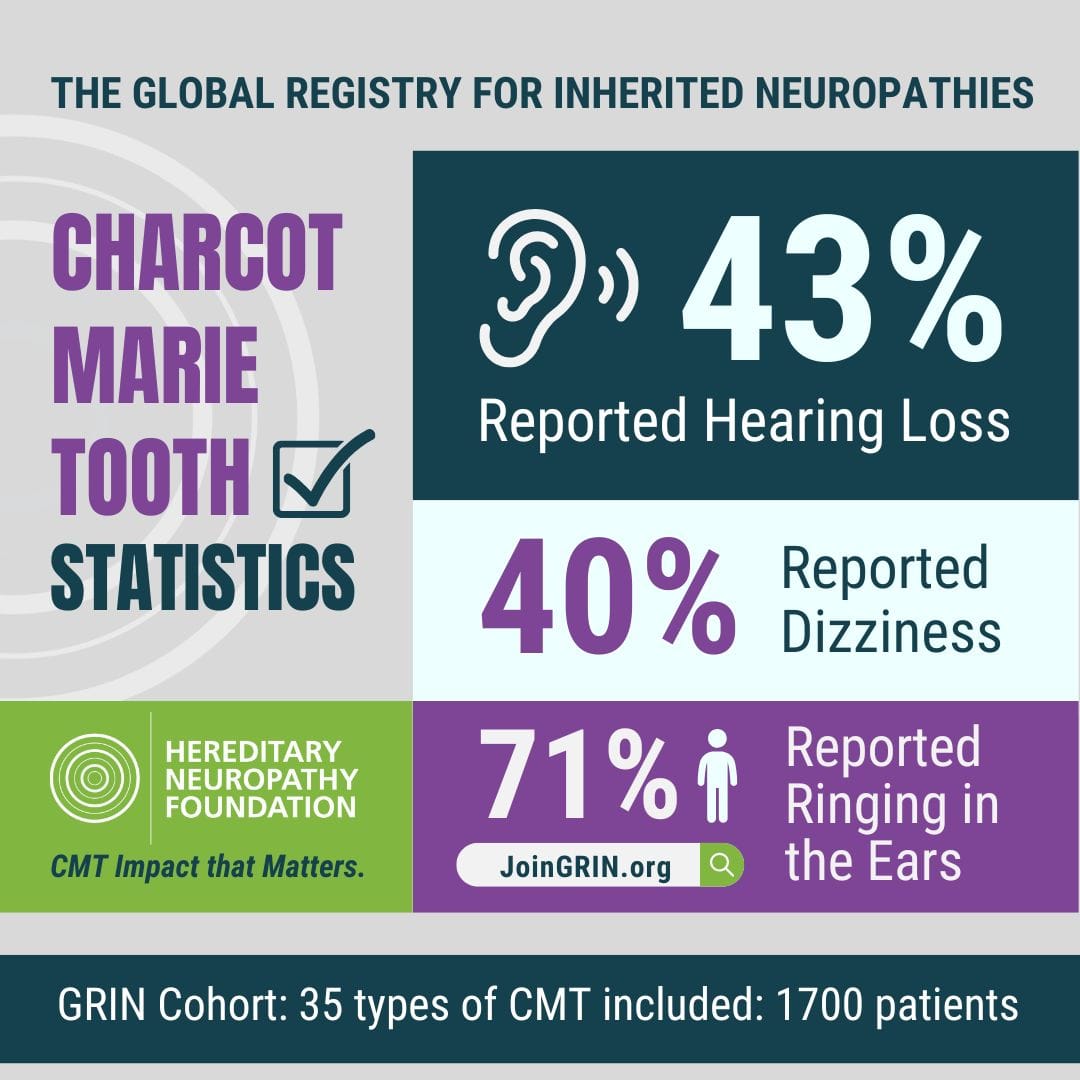
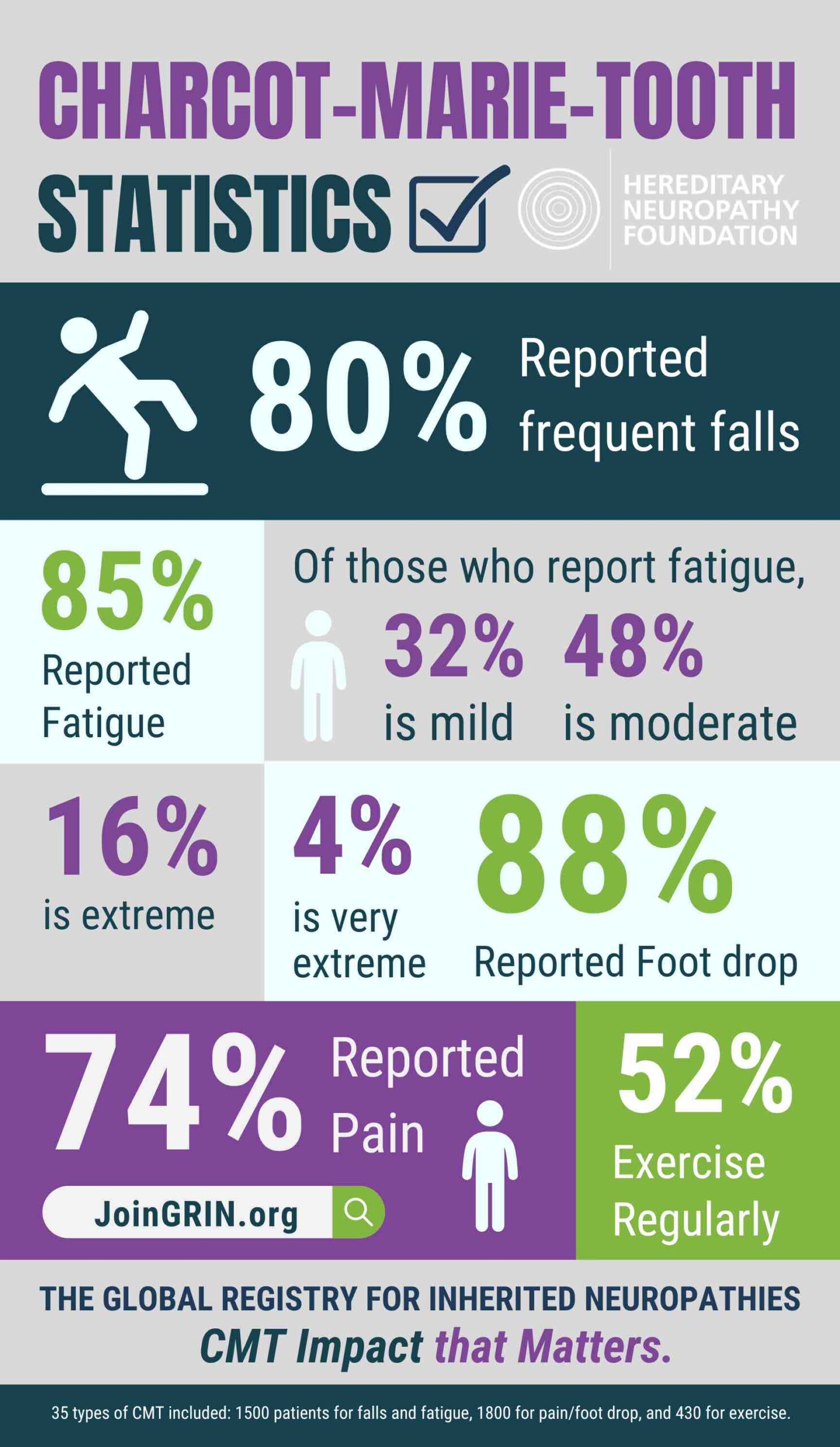




The Hereditary Neuropathy Foundation (HNF), an advocacy and research 501c3 non-profit, today...
Dtx today announced that the FDA has granted Orphan Drug Designation to DTx-1252, an investigational FALCON small interfering RNA (siRNA) therapeutic for the treatment of Charcot-Marie-Tooth Disease Type 1A (CMT1A).
Pharnext announces new positive results from the ongoing open-label extension study of their drug PXT3003
Applied Therapeutics, Inc. has announced that their drug candidate, AT-007 (Govorestat), has received orphan medicinal product designation
Data regarding medical cannabis use for pain relief in CMT from HNF’s CMT Patient Registry, GRIN
The study highlights the significant impact of neuropathic pain on the quality of life and psychosocial well-being of individuals with CMT.
Mitochondria are the powerhouses of our cells. Think of them as our body’s batteries. Mitochondrial disease causes these batteries to run low.
HNF TRIAD Academic Partner Connecticut Children’s Publishes Results of CMT Pediatric Natural...
HNF Awarded 2023 Health Equity in RARE Impact Grant For Spanish CMT PSA Awareness Campaign with Diagnosis & Patient Care Toolkit. The Hereditary Neuropathy Foundation is thrilled to announce that we are a recipient of the Global Genes Health Equity in RARE Impact Grant!
Hereditary Neuropathy Foundation Re-Launches One-of-A-Kind Patient Registry for...
0 Comments