Join the thousands of HNF volunteers who are fueling CMT research. The more you share, the more we’ll learn. Complete your GRIN surveys today!
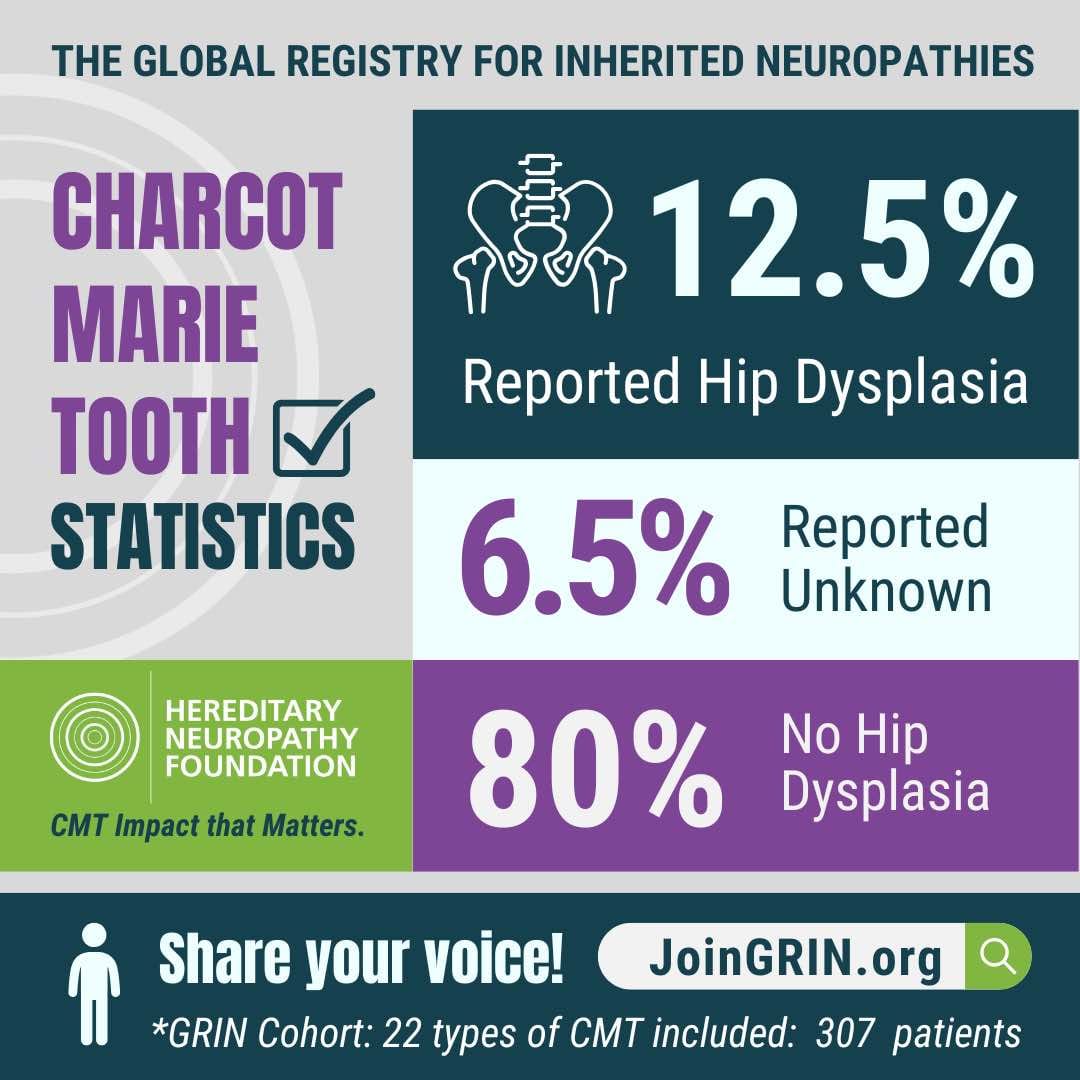
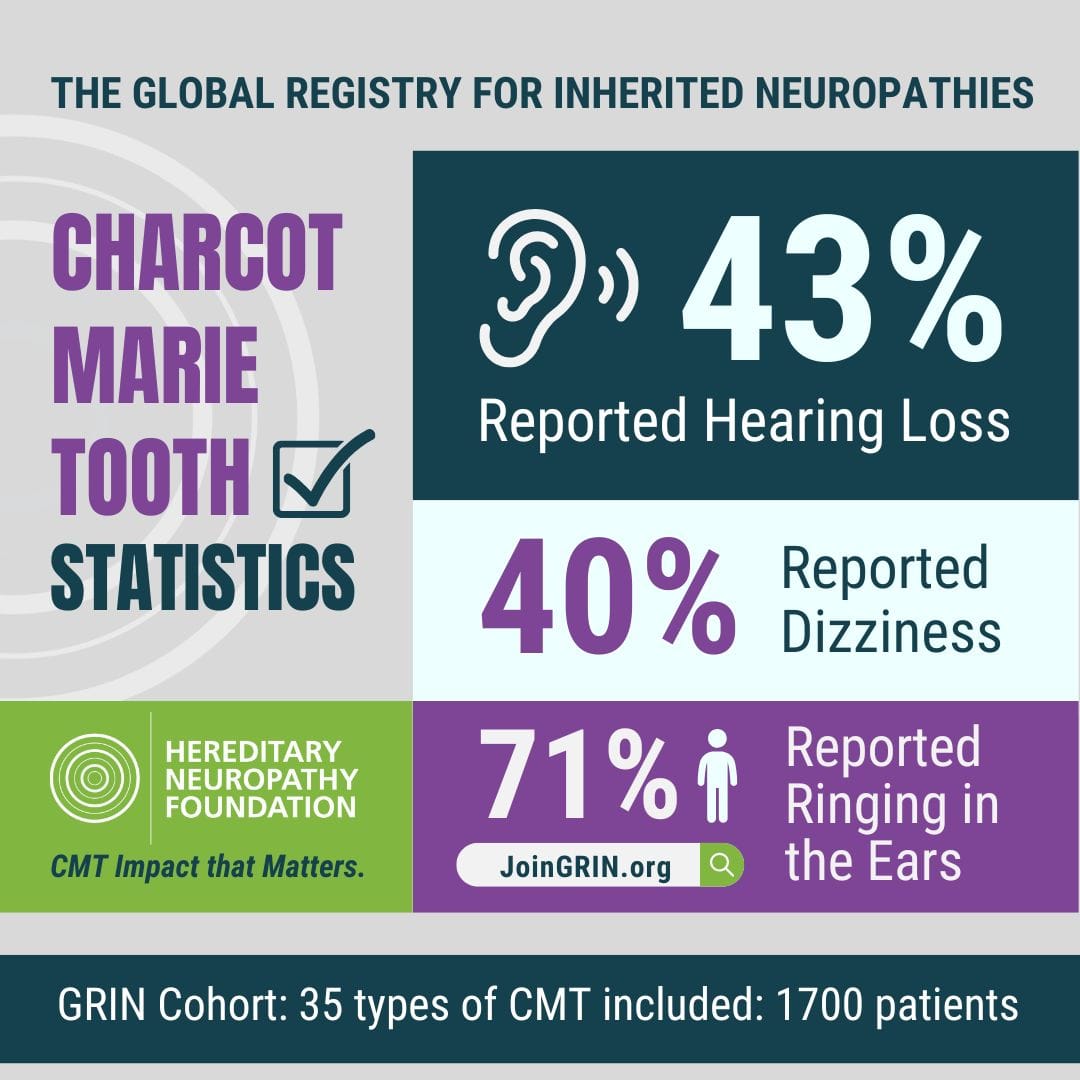
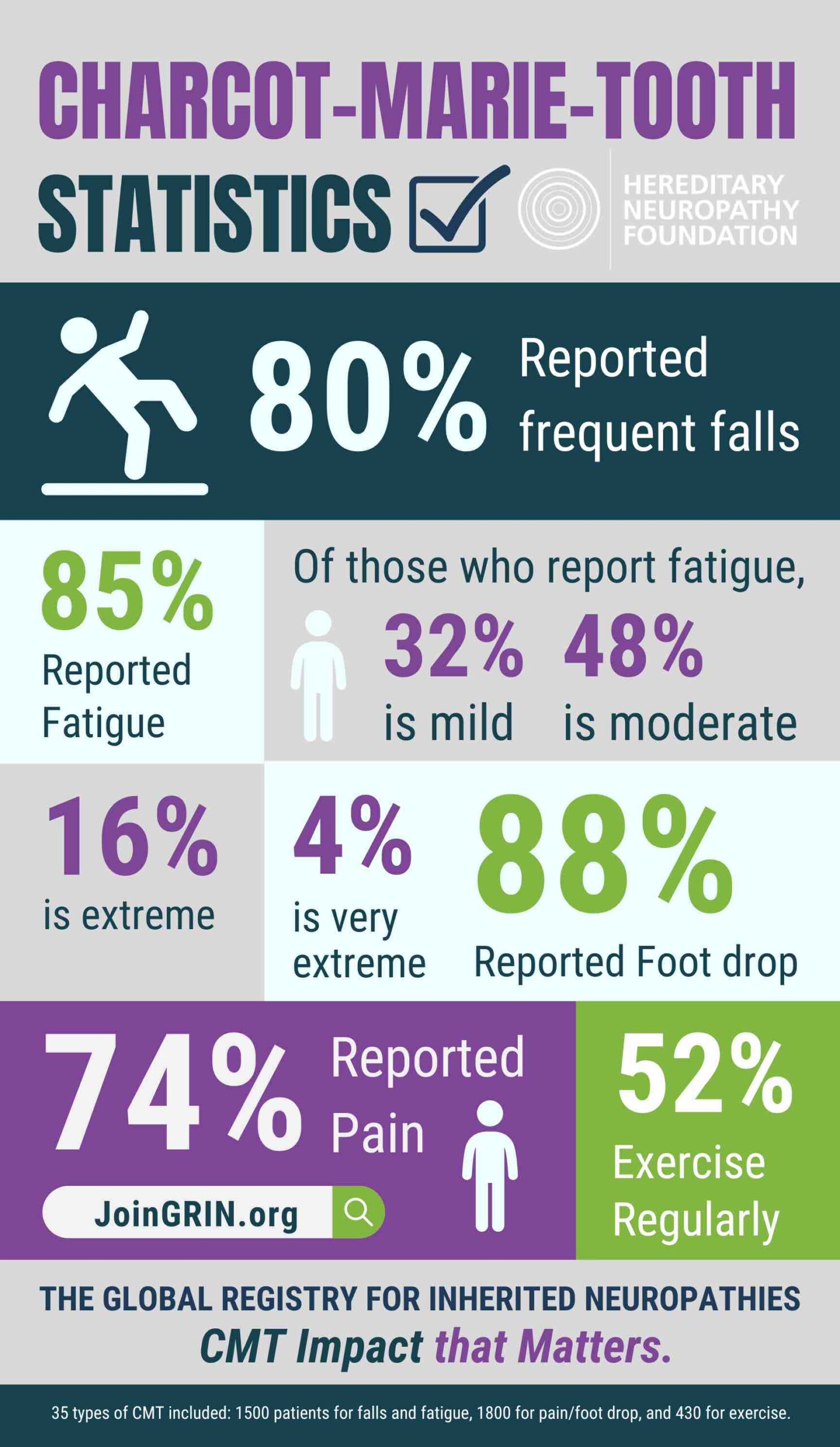




GRIN has played a critical role in identifying important issues related to the CMT patient experience, revealing new areas to explore and research.
Marc Daigle’s CMT story
Rishi’s CMT story
Last summer HNF teamed up with the Penn Medicine Orphan Disease Center for the Million Dollar Bike Ride in Philadelphia.
United Kingdom’s Medicine and Healthcare products Regulatory Agency (MHRA) has granted Promising Innovative Medicine (PIM) designation to its lead drug candidate, PXT3003, for the treatment of Charcot-Marie-Tooth Disease Type 1A (CMT1A) in patients 16 years and older.
Pharnext SA (FR0011191287 – ALPHA) (the “Company”), a biopharmaceutical company pioneering a new approach to developing innovative drug combinations based on its PLEOTHERAPY artificial intelligence platform harnessing big genomics data and network pharmacology, today announced a capital raise of circa € 7.7 million by way of issuance of 1,799,061 new ordinary shares (the “New Shares”) with one warrant attached each (together with the New Shares, the “ABSA”).
Rules and regulations of Wheelchair Rugby
Allison Moore, HNF Founder/CEO, along with her team, took action and led the HNF groundbreaking CMT pain initiative to help the community.
My husband now says he doesn’t want children because of the disease. I am devastated. I always dreamed of a big family.
We want our CMT viewers and listeners to feel like they can relate to us, and that it’s perfectly okay to be imperfect!
0 Comments