Join the thousands of HNF volunteers who are fueling CMT research. The more you share, the more we’ll learn. Complete your GRIN surveys today!
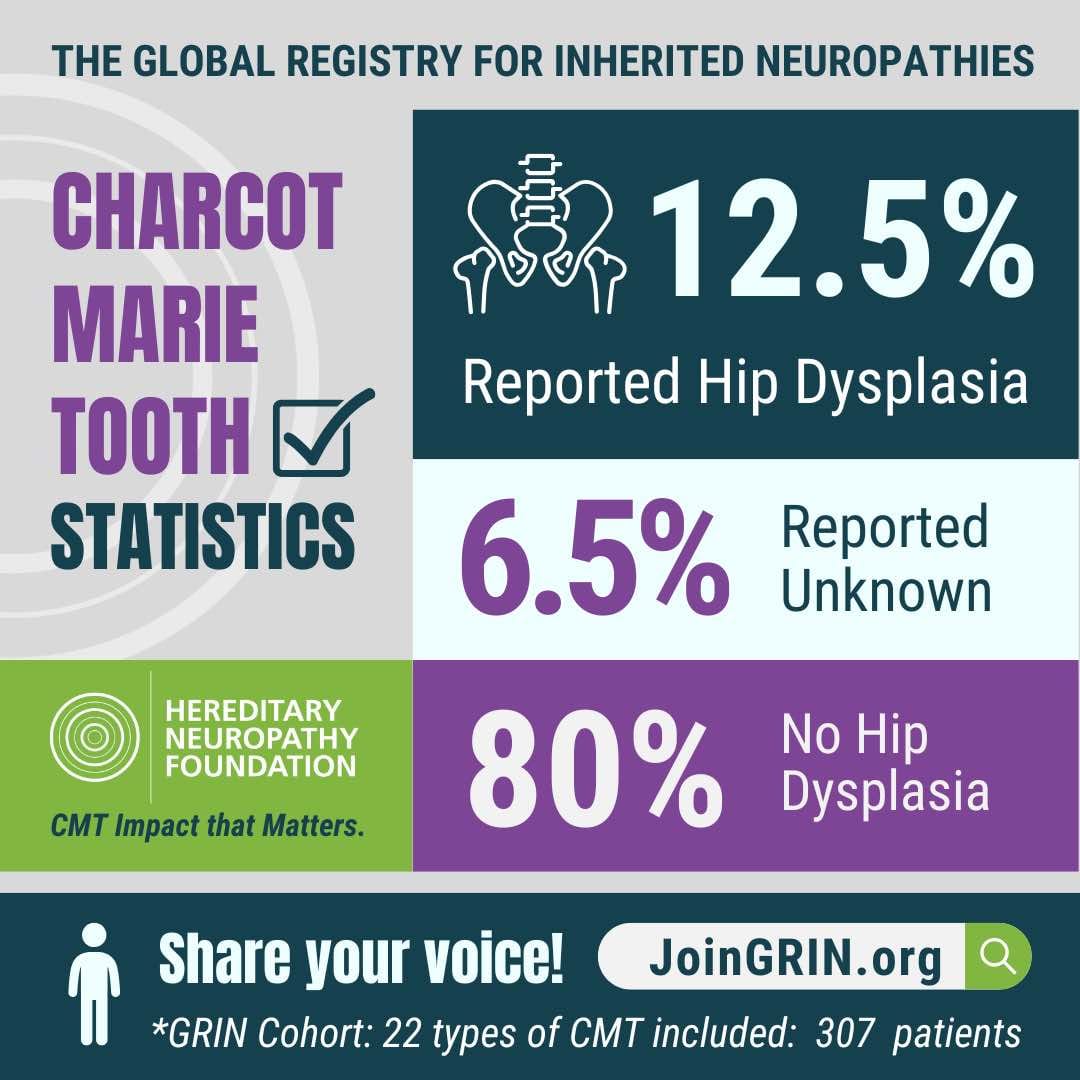
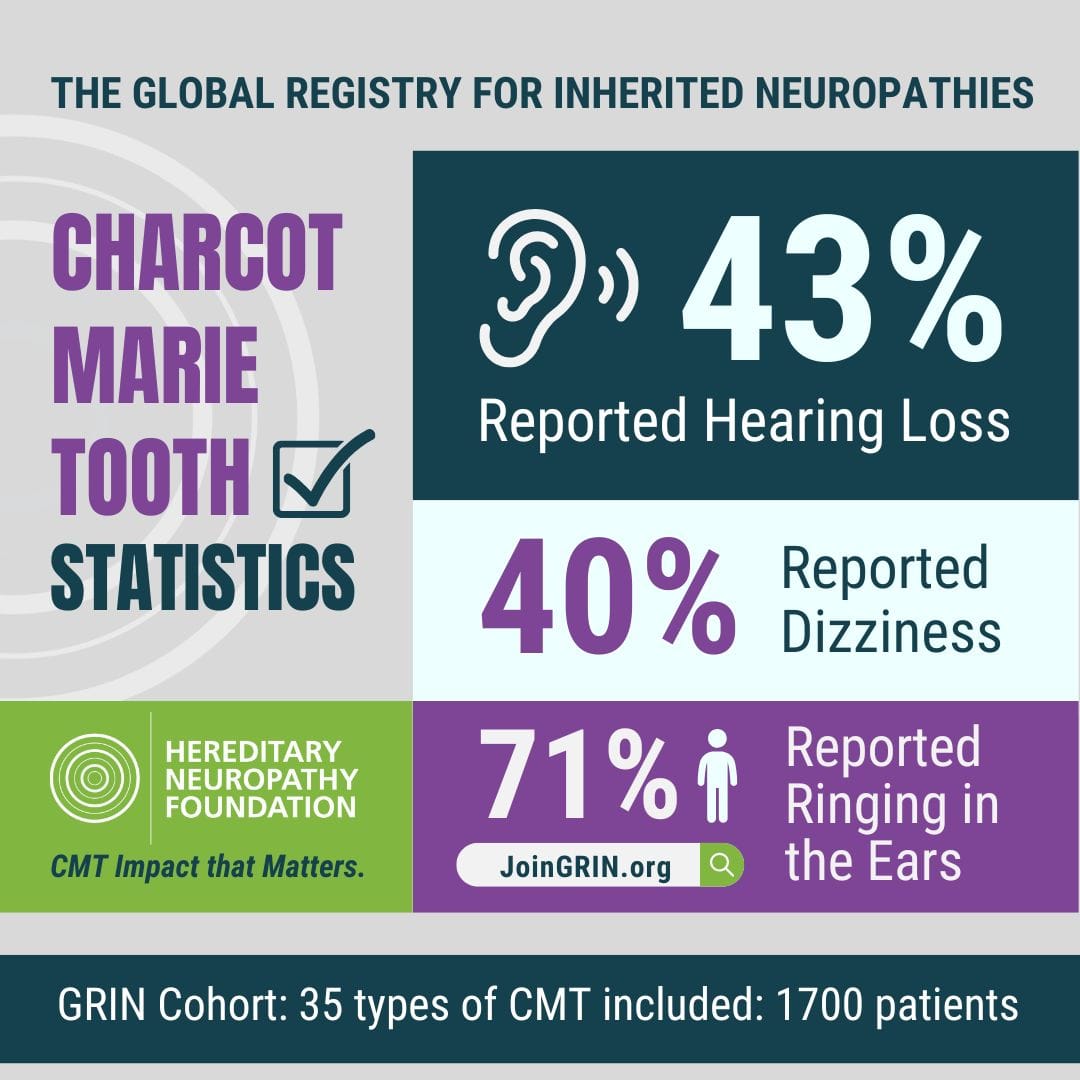
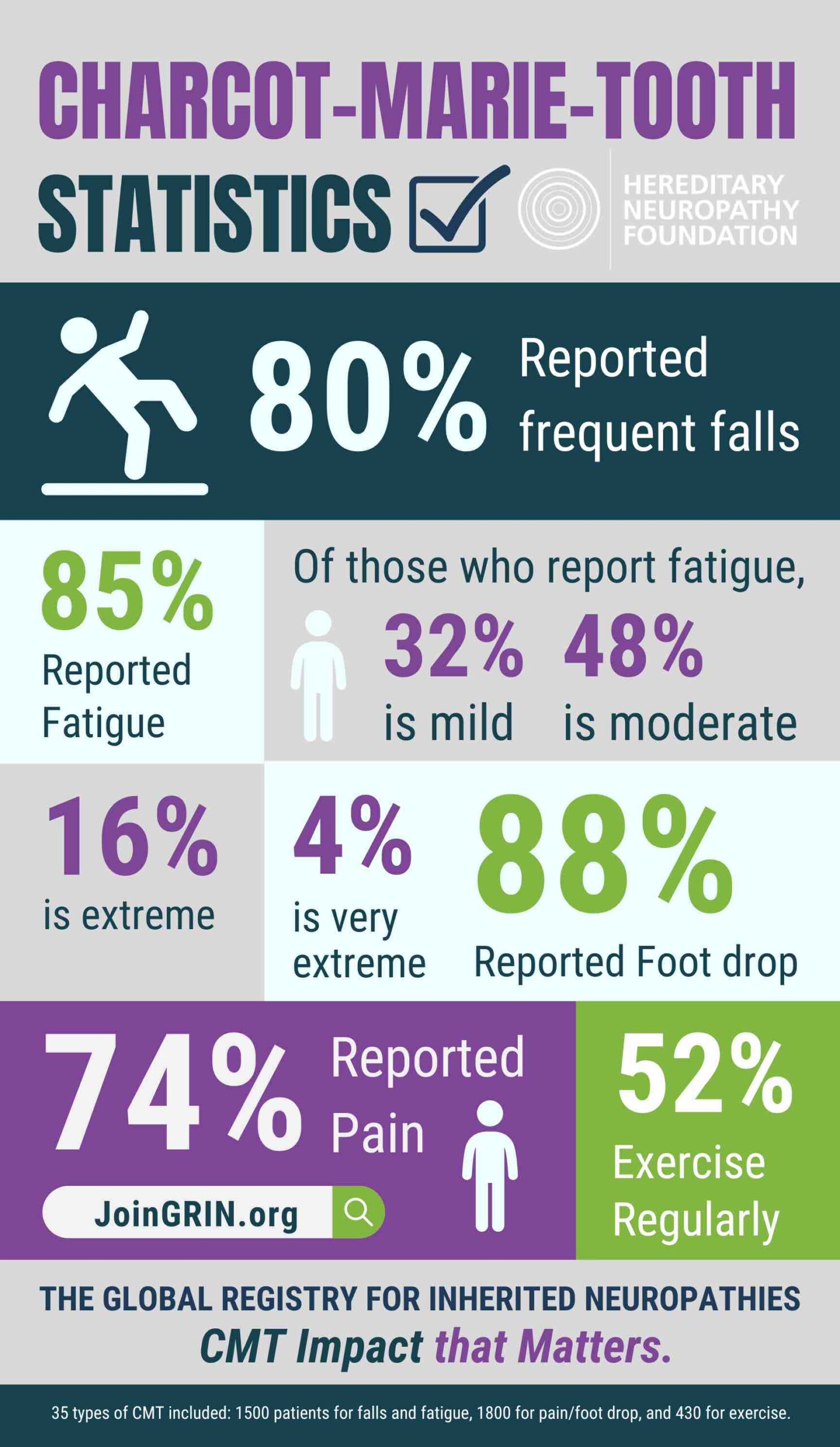




Are you ready to help drive real progress in CMT research? The Hereditary Neuropathy Foundation...
Become a Certified CMT-FOM Clinical Evaluator at the HNF Clinical Trial Readiness Summit The...
[dsm_image_carousel gallery_ids="285782,285783,285784,285785,285786,285787,285788"...
Exciting news for the Charcot-Marie-Tooth (CMT) community! The Hereditary Neuropathy Foundation...
Mark your calendars for April 24–26, 2025, because the Nashville CMT Summit is bringing the...
On March 19th, at the MDA Clinical and Scientific Conference in Dallas, TX, Dr. Cornett will...
CMT Summit + Retreat 2025: Uniting Patients, Researchers, Regulators, and Industry Leaders to...
CMT Simplified is here to make staying informed about Charcot-Marie-Tooth disease (CMT) easier...
NMD670, has been granted Orphan Drug Designation (ODD) by the U.S. Food and Drug Administration (FDA)
CMT Self-Care: A 30-Day Journey to Empowerment and Well-Being Living with Charcot-Marie-Tooth...
0 Comments