“Chronic Pain Assessment: Patient Perspectives”
Allison Moore continues to participate in research initiatives to support CMT patients with chronic pain.
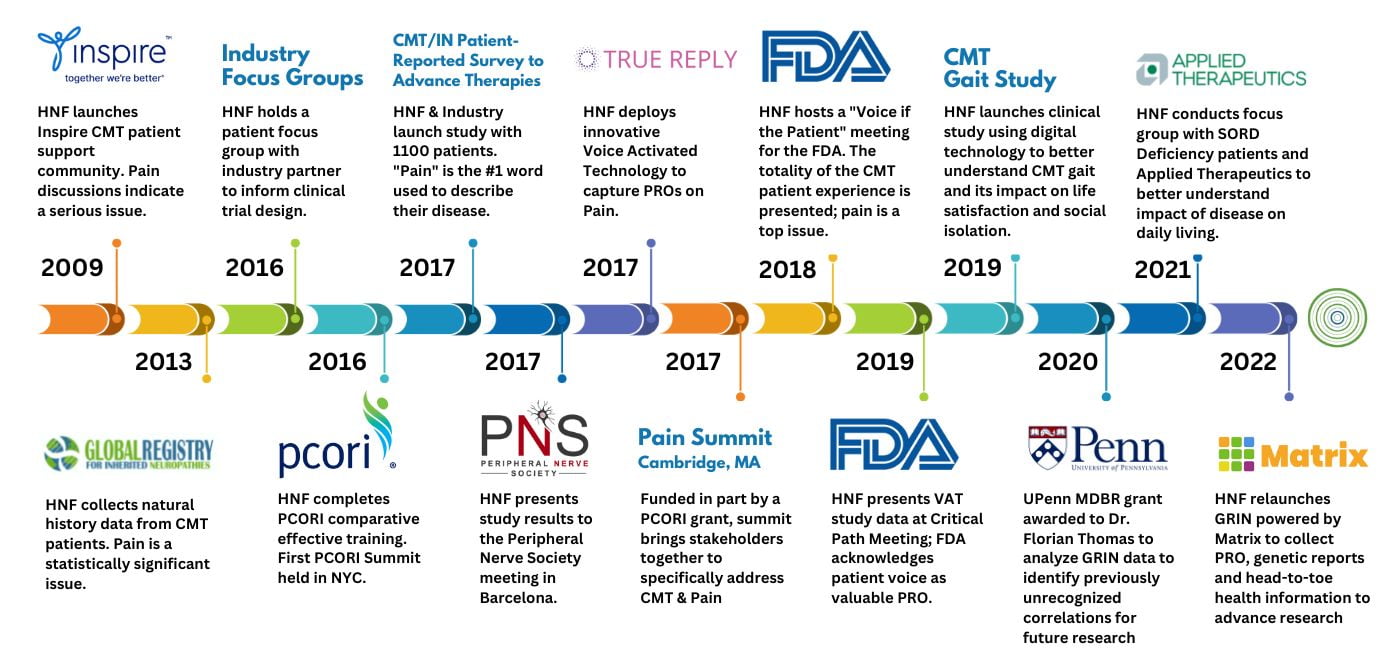
HNF is on the forefront of pain research to support CMT patients. Our team is innovative and strategic, and most of our programs incorporate research efforts with a mission to identify gaps that hinder patient care and to move the needle in bringing treatments to patients.
Joy Aldrich is HNF’s Advocacy Director and moderator of our online Inspire CMT community program (a safe haven for patients and families to communicate and share their experiences of living with CMT). She identified an overwhelming theme of pain amongst community members. She realized that patients and their families are in serious distress due to pain with little to no support from medical professionals or advocacy groups, and were often being told there is nothing to help them.
Allison Moore, HNF Founder/CEO, along with her team, took action and led the HNF groundbreaking CMT pain initiative to help the community.
HNF partnered with Acceleron Pharma and conducted a patient reported outcomes (PROs) research study to capture PROs to inform the Acceleron clinical team with data to design a Phase II, part 1, protocol to treat CMT1A, CMT1B and CMTX. This study has been instrumental to the CMT research community and has increased stakeholder engagement and funds to support critical programs for our patient community!
First, HNF quickly assembled a group of patients at Acceleron’s headquarters in August, 2016 for a focus group to learn more about what matters most to patients when thinking about a treatment. From the initial data collected, they determined that more data was needed, and later launched a Patient-Reported Outcomes (PRO) Study through HNFs Global Registry for Inherited Neuropathies (GRIN). HNF launched the PRO study Charcot-Marie-Tooth (CMT)/Inherited Neuropathies (IN) Patient-reported Survey to Advance Therapies in February 2017. Not only did this study help Acceleron to design their pivotal Phase II, part 2, ACE-083 clinical trial, but it reiterated to us that pain is a huge gap that needs to be addressed for patient care and as an outcome measure in clinical trials.
This study has been groundbreaking. It not only informed Acceleron, but other pharma/biotech, industry, researchers, and the FDA on the impact that pain has on our patient community. Taking additional action, Allison Moore, HNF Founder/ CEO, joined the US Pain Collaborative, comprised of advocacy leaders from across the pain community: the Chronic Pain Research Alliance, For Grace, the International Pain Foundation, Reflex Sympathetic Dystrophy Syndrome Association, and U.S. Pain Foundation. With guidance from Voz Advisors, a biopharmaceutical consultancy, and with funding from Grünenthal Pharma, the collaborative wrapped up this year announcing the publication report, titled Chronic Pain Patients Pinpoint the Need for Improved Methods to Assess Pain. https://prn.to/2sAv7uf
The report is, in part, informed by a 2018 survey designed to understand the patient perspective on how physicians and other healthcare professionals utilize chronic pain assessment instruments. The survey was disseminated to patients through the organizations of several US Pain Collaborative members. Chronic pain survey respondents numbered more than 2,700, and survey results showed that the impact of chronic pain on patients’ lives is not adequately, consistently, or uniformly measured. Over 90% of patients surveyed indicated changes are needed in the way healthcare professionals evaluate chronic pain.












0 Comments