Hereditary Neuropathy Foundation (HNF) is proud to announce that Founder and CEO Allison Moore will participate as a panelist in the inaugural Critical Path Institute (C-Path) Global Impact Conference, taking place from September 9-11, 2024, at the Washington Marriott at Metro Center. The session, titled: “Navigating the Patient-Focused Drug Development Roadmap,” will be moderated by Dr. Cheryl Coon, Vice President of the Clinical Outcome Assessment (COA) Program at Critical Path Institute, and is scheduled for Monday, September 9 at 11:20 a.m.
Additional panelists include:
- Dr. Lindsey Murray, Executive Director of the Rare Disease Clinical Outcome Assessment (RD-COA) Consortium at Critical Path Institute
- Dr. Naomi Knoble, Associate Director of Rare Disease Measurement Science, DCOA, OND, CDER at the U.S. Food and Drug Administration (FDA)
- Dr. Michelle Campbell, Associate Director for Stakeholder Engagement and Clinical Outcomes in the Office of Neuroscience at the FDA.
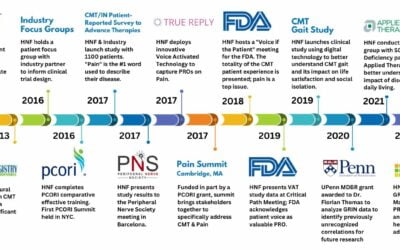
C-Path’s mission is to lead collaborations that accelerate drug development, advancing treatments for people in need worldwide. HNF has partnered with C-Path and its CEO, Klaus Romero, M.D., M.S., to better understand the current barriers and opportunities for FDA approval processes for Charcot-Marie-Tooth (CMT).
This powerful partnership gained international attention at this year’s Global BIO International conference with HNF’s hosted panel, “Revolutionizing Clinical Trials: Patient Registries, Wearable Tech, and Video Capture”, where Dr. Romero was the moderator. Subsequently, he presented to CMT patients, care partners, researchers, and industry at HNF’s CMT Clinical Trial Readiness Summit in San Diego.
CMT is a genetically heterogeneous motor and sensory neuropathy characterized by progressive sensory loss and weakness of the legs, feet, arms and hands causing gait difficulties, frequent falls, balance impairment, and hand weakness.
In February, HNF shared important de-identified patient registry data with C-Path to engage more attention for CMT, including the more rare subtypes. By working together, we hope to eliminate barriers of data sharing, drive more innovation for discoveries and to optimize value in creating new insights and tools that accelerate drug development for CMT.
“Our HNF partnership represents a significant expansion of C-Path’s mission, focusing on critical areas of drug development. These areas highlight how C-Path’s areas of core excellence — data management and standards, biomarkers, modeling and analytics, regulatory science, and clinical outcome assessments — interlink across all stages of drug development, demonstrating the value of C-Path’s public-private partnership model.” – Klaus Romero, M.D., M.S., C-Path CEO
HNF encourages the patient community and industry working on CMT to attend and gain firsthand knowledge and actionable guidance from the panel discussions with regulatory experts at the forefront of rare diseases and drug development.
“This meeting represents a critical milestone in HNF’s step #4 in our “Roadmap to CMT Therapies.” We are right on track and gaining momentum each step of the way.” – Allison Moore, Founder/CEO, HNF
This event is free to all CMT patients and care partners and is a great opportunity to share their voices and connect with key decision-makers. To learn more and *register, visit: https://web.cvent.com/event/e25a101c-7408-4295-b318-4073790c6f27/summary
*Registration closes August 31, 2024 at 4:59pm ET.
About the Critical Path Institute
Critical Path Institute (C-Path) is an independent, nonprofit established in 2005 as a public-private partnership, in response to the FDA’s Critical Path Initiative. C-Path’s mission is to lead collaborations that advance better treatments for people worldwide. Globally recognized as a pioneer in accelerating drug development, C-Path has established numerous international consortia, programs and initiatives that currently include more than 1,600 scientists and representatives from government and regulatory agencies, academia, patient organizations, disease foundations and pharmaceutical and biotech companies. With dedicated team members located throughout the world, C-Path’s global headquarters is located in Tucson, Arizona and C-Path’s Europe subsidiary is headquartered in Amsterdam, Netherlands. For more information, visit c-path.org. FDA Acknowledgement










0 Comments