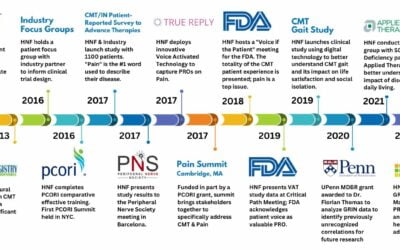
Daniel Levine from Global Genes spoke with HNF’s CEO and Founder Allison Moore on this edition of Global Genes Rare Cast about how HNF used a technology company to gather patient information to present at the Patient-Focused Drug Development Meeting with the FDA.
CMT can impact the ability for patients to type or write. By joining forces with a technology company, HNF was able to gather patient feedback using voice technology, allowing patients to give their comments over the phone through a simple automated system.









Love what you’re doing and your progress. I don’t know how, other than politically, to help get Pharnext approved in Canada. Obviously it is easier once a drug has FDA Approval. I have a son who could be in clinical trials. I had DNA Testing which confirmed I have CMT1A so we assume he does also. Will the drug be affordable in the US and Canada?